
Integrated microwave photonics
Tap into imec’s optical beamforming platform to develop directional and high-bandwidth communication hardware for the next generation of mobile networks.
Microwave photonics combines two worlds: those of radio frequencies and photonics. It attracts interest from both the research community and the commercial sector for its ability to create functions that are very complex and power-hungry, or not directly possible in the RF domain. For example:
- broadband and flexible beam shaping and beam steering via phase shifting
- frequency conversion at a wide RF spectrum – up and beyond 30 GHz
In particular for beamforming networks, imec developed a technology platform that enables multifunctional and high-performance circuits through hybrid integration of microwave photonics components. Now you can gain access to this platform to build hardware components tailored to your applications.
Value of optical beamforming for telecom networks
At the heart of networks for 5G and beyond are beamforming devices that enable directional and high-bandwidth communication links. These devices need to be compact, low-power, and resilient to interference to create flexible bandwidths.
Optical beamforming is the preferred technology for the development of these devices. It allows true time delay from one picosecond to a few nanoseconds. That’s not possible with RF-based beamforming, and is extremely power-consuming with digital beamforming.
Hybrid integration of microwave photonics
As an advanced R&D hub with deep knowledge of materials, circuits, and systems, imec can create optical beamforming devices for telecom that combine the best of two technologies:
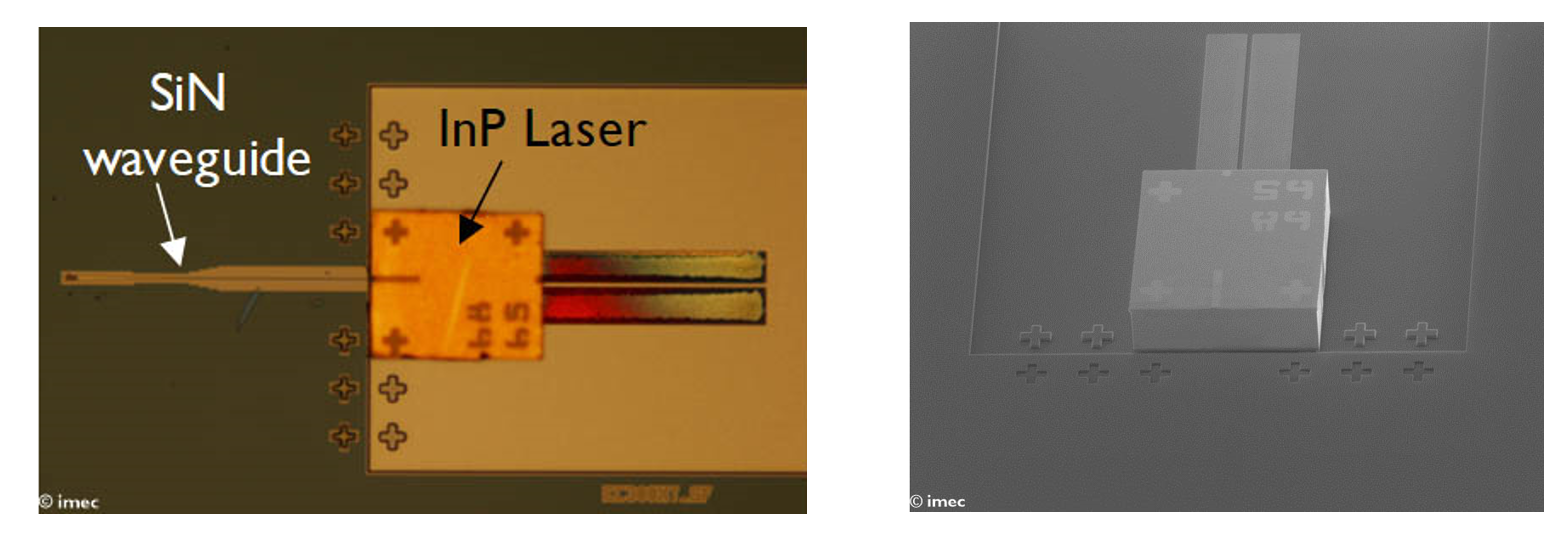
- Indium Phosphide (InP) for Mach-Zehnder interferometers (MZM), lasers and detectors
- low-loss silicon nitride (SiN) for switches and delay channels
An example is the addition of lasing and amplification to a passive SiN platform by flip-chip bonding of the laser to a SiN waveguide.
Want to discuss the possibilities of integrated microwave photonics for your application?
