The best-known example of an autonomous and closed-loop therapy device is a pacemaker. Second in line, although not yet commercially available, are intelligent insulin pumps. With artificial intelligence, and miniaturized sensors and actuators, the ingredients are there to shape a future with a multitude of closed-loop systems for remote, preventive and curative healthcare.
Think of medical-grade wearables measuring blood pressure and assisting in finding the right dose of blood pressure-regulating medicine. Think of small implants that stimulate specific nerves based on biomarkers for migraine sensed in real time. Or think of invisibly integrated sensors in (car) seats and office chairs to follow up the breathing capacity of lung patients and advise them on their fitness program.
In this article, Vojkan Mihajlović, senior researcher, and Evelien Hermeling, principal scientist at imec, share their vision on how closed-loop systems, consisting of wearables, implantables, invisibles and smart algorithms, will transform healthcare.
From pacemakers to anesthesia
Google ‘closed loop in healthcare’ and you immediately get thousands of hits on the artificial pancreas. Evelien Hermeling: “In fact, the evolution of diabetes care is ideal to illustrate what a closed-loop therapy system is. Patients moved from finger pricks and insulin pens to glucose monitors on their abdomen or upper arm that measure continuously and send alarms to their cellphones.”
“More advanced systems today (such as the Minimed™ 670G Insulin Pump System of Medtronic) go one step further and add a small wearable computer to the continuous glucose monitor. That computer captures the data, calculates how much insulin is needed and sends this info to the insulin pump that is also attached to the patient’s abdomen. In this way, blood sugar remains steady day and night, automatically. That vision is pursued for other diseases as well, to make the lives of patients healthier and more comfortable. The artificial kidney for dialysis patients is another typical example, although still far in the future. Imec, together with leading research organizations in the field, has and will continue to work on a roadmap to make such an artificial kidney a reality.”
It is also forecasted that for other chronic or acute conditions, next-generation treatments will no longer be restricted to chemicals. Doctors might soon be able to prescribe drug treatments in combination with devices that monitor the effect of the drug or suggest changes in its dosage (medically known as titration process). These kinds of treatments are known as drug-device combinations.
Vojkan Mihajlović: “Also for doctors, closed-loop systems are there to make life easier. Think of a closed-loop system for anesthesia: the anesthesiologist sets a target blood pressure and the system automatically maintains this target by dosing the appropriate medication. This allows specialists to focus on the patient and relieves them from repetitive, automatable tasks.”
Compose your future closed-loop therapy system
With the advent of miniaturized, wireless, reliable sensor and actuator systems, and artificial intelligence for interpretation of the data, the time has come to fully invest – both money and brains – in new systems that are smarter and more autonomous when it comes to keeping an eye on patient’s health.
Evelien Hermeling: “A typical closed-loop system is made up of different key parts, each with its specific requirements. The sensing part measures one or more parameters of the patient’s body, such as heart rate, ECG, blood pressure etc. Sensors must be accurate, small, and able to wirelessly transmit their data to the cloud or a device in the patient’s vicinity.”
“Next, a small processing unit is needed, executing algorithms that can interpret these sensor data into actionable insights. Depending on the use cases, time constraints need to be considered. For example, a heartbeat needs to be acted upon immediately (like with a pacemaker), while blood pressure is known to take time to change and here it is more important to watch a trend over a few hours or even days. Also, personalization is key. 50 beats/min may be a normal resting heart rate for one and an abnormally low heart rate for someone else. Algorithms need to add this kind of intelligence to the system so that useful and accurate actionable insights can be deducted from the sensor data. Next to time constraints and personalization, also contextual and environmental parameters need to be considered. Blood-pressure-lowering drugs, optimally titrated to a person for an average day, might cause the blood pressure to drop during hot weather. This can be a dangerous situation that needs to be corrected. Algorithms would be able to adjust the doses, taking such contextual and environmental parameters’ into account.”
“A third building block is the control algorithm, that translates the insights into a control action. For example, too much fluid buildup in the body can result in increase in medication dosage for a patient. Again, personalization and time constraints are key here. Imagine you are dealing with diuretics (pills to help increase urine volume), then the control algorithm should accommodate that the body needs some time to get rid of the excessive fluids. Delivering a medicine in the bloodstream will not immediately change the fluid status.”
“And the last part is the actuator hardware, e.g. the micropump delivering a drug into the blood stream, based on the instructions it received from the control algorithm. A small form factor, and accurate and timely operation are the most important features of this building block.”

Key building blocks of a closed-loop therapy system. The dark blue ones are typically hardware components, while the white-colored blocks are mainly software modules.
These closed-loop therapy systems can have many different form factors: wearables, implantables, invisibles, ... Vojkan Mihajlović: “Invisibles are sensors that are seamlessly integrated into the patient’s environment. Think of sensors in a (car) seat, a car’s dashboard, a mattress or even a toilet seat. While wearables and implantables measure continuously, invisibles only measure occasionally, which is sufficient for certain types of measurements. Think for example of a daily check of blood pressure, urine composition or lung capacity.”

Closed-loop therapy devices can take on many forms. Think for example of medical-grade wearables measuring blood pressure and assisting in finding the right dose of blood pressure-regulating medicine.
Imec has all the building blocks in house
Imec is known for its work on miniaturization of electronics, which is researched in state-of-the-art cleanrooms. Evelien Hermeling: “Some 15 years ago, work started on sensing systems for vital sign monitoring. This work resulted in collaborations with industry leaders such as Samsung and Biotelemetry, in a large study on stress monitoring in real-life conditions, and in different prototype devices for measuring brain waves, eye movements, gait, sleep patterns, respiration, pain and stress, and general health parameters.”
“The focus has been on making very compact, low-power and wireless sensor systems, with efficient data acquisition, using different modalities (activities of the heart, lungs, muscles or nerves) and integrating them in different form factors (watch, patch, headset, ...). We are now working towards expanding the efficient acquisition portion, with smart algorithms to provide actionable insights, and actuation principles to close the loop.”
“We invest a lot of work into algorithm development for good interpretation of the collected sensor data and to ensure reliable data quality in real-life conditions and in uncontrolled environments. Also, algorithms allow the fusion of all available sensor data, from different kinds of sensors, in order to draw even more reliable conclusions.” In this article on respiratory monitoring, the use of algorithms (signal-level, digital-biomarker-level and application-level) is further explained.
Vojkan Mihajlović: “In the domain of actuation and stimulation, imec explores the possibilities of non-invasive brain stimulation (with its EEG headset) and electrical stimulation of nerves with small implantable nodes. Also, we are developing a general-purpose software framework that facilitates multimodal data acquisition, signal interpretation and specification of control rules required for optimal actuation. This framework links all these ingredients into a true closed-loop system. The development currently focuses on e.g. timing requirements and feedback/actuation modalities. Of course, imec looks for partners and users to complement this expertise, especially from the application side.”
Below are 3 examples of work that imec has done over the years related to closed-loop systems. As said, closed-loop feedback systems can be embodied in multiple form factors, such as wearables, implantables and (non-contact) invisibles or combinations of these. For each of these form factors, we highlight one example. At imec, we specialize in using our knowledge and experience to solve customer-specific challenges, hence the following examples are meant as a source of inspiration. Each application requires different building blocks used in different manners.
1. A headset that combats depressions, migraines, ...
Transcranial current stimulation (tCS) relies on injecting currents (a few mA) over the human scalp in order to modulate ongoing brain activity.
Vojkan Mihajlović: “The applications for tCS are numerous, ranging from treatment of depression and migraine to epilepsy and anxiety disorders. In a closed-loop system, it is important to measure the brain waves while and after applying stimuli, such that the stimuli can be adapted to facilitate personalized treatment.”
"Traditionally, the method involved two large electrodes placed at different locations on the scalp with current going from one electrode to the other. The electrodes were made of conductive polymer enclosed in a sponge-like housing, soaked in a saline solution."
“More recent approaches of tCS rely on a larger number of smaller (1 cm²) electrodes. Imec developed EEG monitoring headsets with small dry electrodes (together with Datwyler). They are very easy to use and deliver high-quality measurements. Currently, such dry electrodes are being adapted for tCS. For this application, imec also plans to extend the headset’s capabilities with digital active electrodes, with both electrical and optical features. By using two different modalities, it becomes possible to simultaneously measure and stimulate without interferences (e.g. electrical stimulation, optical sensing and vice versa).

Imec’s EEG headset research platform with optical and electrical modalities to both measure and stimulate brain activity. Possible applications are treatment of depression, migraine, epilepsy, ... Imec looks for partners to work out a system for specific applications.
Imec developed a phantom head to facilitate the exploration of safety aspects and the impact of tCS on head tissue. Also, it allows to optimize closed-loop brain monitoring and stimulation. This phantom head is the electrical equivalent of a human head consisting of different head tissue layers. It enables probing the current paths at different surface locations and tissue depths. Tests done using brain monitoring and tCS systems applied on the phantom head, along with simulated EEG signals (generated by using signal generators), are a steppingstone to closed-loop EEG & tCS trials in humans.
Watch the video below on imec’s explorative platform for personalized, non-invasive transcranial current stimulation:
2. Small implantable nodes that tickle your nerves
Next to wearables, implanted devices can also do sensing and actuation of vital sign parameters as part of a closed-loop therapy system. Vojkan Mihajlović: “An important field, with an enormous potential, is bioelectronic medicine, in which the central or peripheral nervous system is electrically stimulated. Stimulation can be done in the brain region, the spinal cord, or the Vagus, hypoglossal, phrenic, sacral or tibial nerves.”
“In the brain, microneedles are the preferred form factor whereas tiny stimulation nodes placed next to nerves or miniaturized cuff electrodes wrapped around the nerve bundles, can be used in the rest of the body. Imec develops both neuroprobes and small stimulation nodes. With both form factors, the focus is on miniaturization and wireless powering and communication.”
An important milestone, end 2018, was the release of a neural microneedle probe, Neuropixels, to the global neuroscience research community. The probes were developed through an international collaboration funded by the Howard Hughes Medical Institute (HHMI), Wellcome Trust, Gatsby Charitable Foundation and Allen Institute for Brain Science and designed, developed and fabricated at imec, in collaboration with HHMI Janelia Research Campus, Allen Institute for Brain Science, and University College London. The probes have almost a thousand electrodes and 384 recording channels on a single shank, providing an unprecedented resolution for mapping brain activity. Now, first steps are being taken in extending the Neuropixel platform towards stimulation.
Vojkan Mihajlović: “Another example, with a different form factor, are the implantable stimulation and sensing nodes that we developed and that allow the stimulation of human tissue and at the same time capture impedance and temperature. They are powered and communicate using wireless protocols, hence they can be deployed in the human body for an extremely long time.”
“You could also combine implantable nodes with wearable devices. Think for example of an external wearable sensor that measures heart rhythm, respiration or digestive activity from the skin surface. This would then communicate with the implanted neurostimulators that counteract any sensed abnormality.”
Imec develops both microneedles and implantable nodes for electrical sensing and stimulation of nerve cells.
3. Use the patient’s environment to monitor vital signs
Next to, or in combination with wearables and implantables, one can also use sensors in the environment to monitor patient’s vital signs, at specific moments of the day. Miniaturized sensors can be integrated in chairs, car seats, beds, toilets etc.
Evelien Hermeling: “Imec explores capacitive, optical and radar technology to do vital sign monitoring ‘from a distance’. For example, in a car one could build sensors into the driver’s seat, steering wheel and dashboard to measure respiration rate, blood pressure, heart rate and cardiac activity.”
“As a demonstrator, we integrated capacitive sensors in an office chair and a car seat to carry out ECG readings and detect respiration rates through clothing. This principle is not new, but the technology has not been used before in practical applications because the quality of the readings was poor due to movements of the person in the chair.”
“The solution lies in the use of smart algorithms. First, algorithms can compensate for variations when movements and artifacts are detected, which makes the readings more reliable. Second, algorithms can make the system adaptive. This means that, in good conditions, an (ECG) signal of medical quality can be recorded. When conditions are not so good, the sensors switch to robust mode and take more general readings. For example, although you can still record the heart rate, obtaining an accurate ECG graph is not possible. This variable quality is factored into the readings and passed on as such – together with the results recorded.”
Such sensing information could for instance be used as input to dynamically and automatically adapt the driving conditions and the level of self-steering of the car, or to generate actionable alarms.
Capacitive sensors can be incorporated into an armchair, bed, office chair or car seat. Imec has developed a system that can support up to 64 sensors. If you want to use capacitive sensors to record respiration rates, it is important in practical applications to make a record of the reliability of the readings as well.
Vojkan Mihajlović: “It’s also possible to use wearables to capture how our senses react to different stimuli in the environment and use this info to adapt the environment. For example, we are collaborating with one of the largest South Korean cosmetics companies, Amorepacific, on capturing user reactions to different fragrances. Based on this, a real-time selection of preferred fragrances is performed. Also, we are setting up a European project in which an avatar adapts its interaction with a real-life person to this person’s emotional state. The possibilities are enormous.”
Future vision: closing the loop with nanobots
Evelien Hermeling: “These are indeed some of the possible future applications of closed-loop therapy. At imec, we have a vision on how these closed-loop systems could evolve (see figure below). In the near future, we foresee a multitude of closed-loop therapy examples using wearables or sensors in the environment, communicating with e.g. one’s smartphone and doctor, resulting in an action to be taken by the person itself. Image your smartwatch measuring your blood pressure and alerting you via your smartphone to take one extra pill.”
“In a next phase, the sensors and actuators could be implanted in the body, so the closed-loop system would be an integral part of the patient, regulating blood pressure etc. We could think of ‘nanobots’ inside the body detecting certain abnormalities (e.g. atherosclerotic plaques inside blood vessels) and a doctor injecting some form of a smart cell to fix the problem. Or could this one day be executed in a completely autonomous way, giving a whole new meaning to preventive medicine? At imec, we like to think big, and lay out truly visionary roadmaps to inspire our researchers and to fuel the discussions with our partners.”
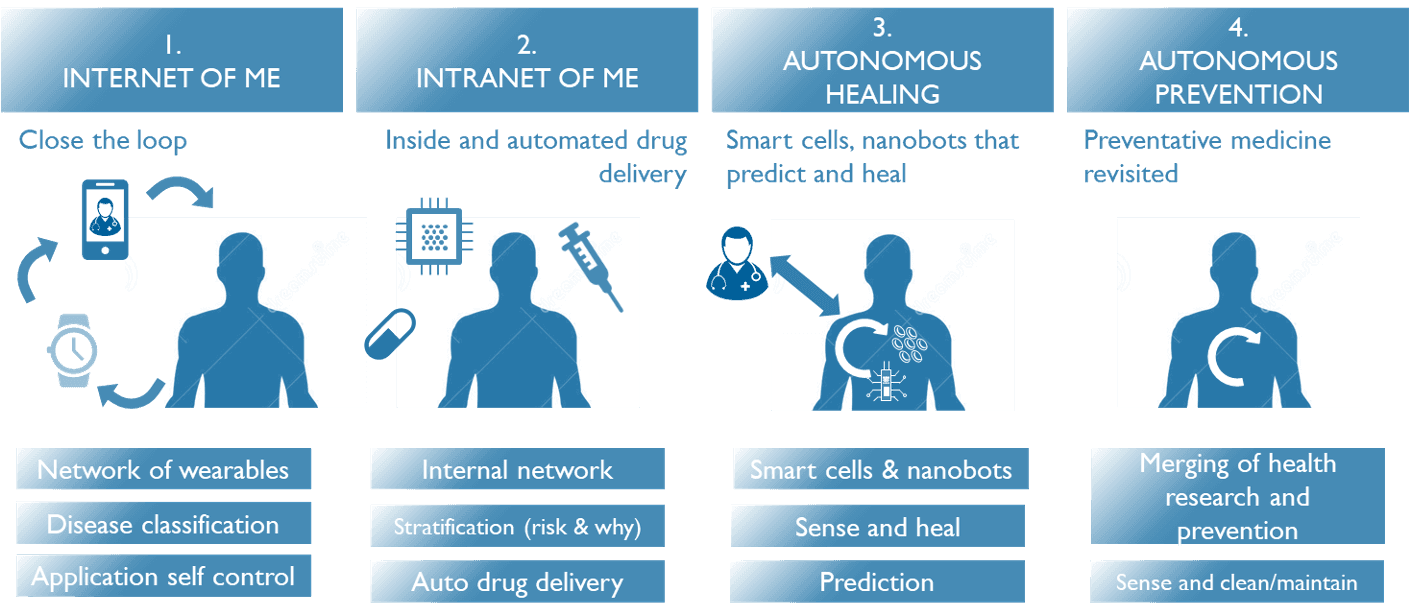
Imec’s future vision on the evolution of closed-loop therapy systems.
Conclusion
Thanks to the miniaturization of sensors and actuators, and thanks to the enormous progress made in artificial intelligence and deep-learning algorithms, it becomes possible to close the loop on health: not only sensing vital signs but also interpreting the data, getting actionable insights, and triggering some action.
This leads the way to artificial organs, drug-device combinations, the targeted treatment of depression and chronic pain, and many other applications. Every closed-loop therapy system will look different and will rely on (a combination of) different form factors (wearable, implantable, invisible) and building blocks to find the perfect fit for a specific application.
Imec researches and develops the key building blocks for such a closed-loop therapy system of the future. Together with application and research partners, this technology can be translated into a tailored solution for specific patient conditions. Partner with us to shape this future!
Want to know more?
- Download our white paper on implantables.
- Learn more about imec and about the different ways to collaborate in the field of connected health solutions.
- Discuss your ideas with Zohaib Gulzar, business development manager

Vojkan Mihajlović is a senior researcher at imec. He received his Ph.D. degree from the University of Twente, Enschede, The Netherlands and has worked as a senior scientist at Philips Research from 2006 to 2012. Since 2012 he has been working on system level and biomedical algorithms at imec. He has extensive experience and leads research activities in the area of wearable brain monitoring and noninvasive neuromodulation. He is also coordinating connected health solution innovation activities.

Evelien Hermeling received her PhD degree in 2009 at the Maastricht University, the Netherlands. After a few years of post-doc at Maastricht University, she started as senior researcher at imec in 2015 and became principal scientist in 2019. Her drive is to bridge the gap between hardware-, software- and biomedical engineers and the medical doctors. She is leading the competence team biomedical algorithms and models within imec-Netherlands.
Published on:
21 August 2020